45 fda structured product labels
Selection of Appropriate SPL Codes for Human Prescription Drug Labeling when creating spl, fda encourages application holders to select the most specific, appropriate logical observation identifiers names and codes (loinc) to allow spl users to identify content within... RxNorm Release Notes - nlm.nih.gov FDA Structured Product Labels: MTHSPL: MTHSPL_2022_05_26: FDB MedKnowledge (formerly NDDF Plus) NDDF: NDDF_2022_05_11: RxNorm normalized names and codes: RXNORM: RXNORM_20AA_220606F: Veterans Health Administration National Drug File: VANDF: VANDF_2022_04_29: The following sources have not been updated since the previous RxNorm release:
Consumer Medical Devices | Consumer Healthcare Products Association - CHPA They have established safety profiles and are for use by consumers in non-clinical environments. Generally, no training is necessary to use a CMD. Manufacturers of CMDs rely on labeling, design, and consumer familiarity to ensure safe and effective use. Most CMDs are not subject to FDA's premarket review requirements. Select an Issue

Fda structured product labels
Senior Regulatory Specialist at Arbonne | JobEka.lk Prepare drug listings for otc drug products for fda structured product labeling (electronic submissions required by fda). Prepare product notification or pre-market approval submissions in other markets for line extensions and new product releasesThese requirements may include current markets such as canada, australia, new zealand, european ... Tech | Best-In-Class Information-Based Solutions and ... Navigator™ for Drug Labels helps pharmaceutical regulatory researchers ensure accurate and up-to-date drug label content. Structured Product Labeling Services for Rx, OTC and Biologics Manage product listing, establishment facility, and labeler company data across multiple teams with a secure cloud-based solution. 0078-0380-05 | Dexmethylphenidate Hydrochloride | National Drug Codes ... 0078-0380-05. The labeler code, product code, and package code segments of the National Drug Code number, separated by hyphens. Asterisks are no longer used or included within the product and package code segments to indicate certain configurations of the NDC. Package Description. 100 TABLET IN 1 BOTTLE (0078-0380-05)
Fda structured product labels. › media › 121326U.S. Food and Drug Administration 2015-10000 12/17/2015 Closed LATHAM & WATKINS LLP 11104397 11063344 10989897 ETC 2015-10001 Steptoe & Johnson LLP FAP 2258 2015-10002 HemoCue AB K142209 EOF THE FOOLING OF AMERICA CONTINUES: An Update on Availability of ... At that time, the FDA published a BLA package insert that included the approved new COVID-19 vaccine tradename COMIRNATY and listed 2 new NDCs (0069-1000-03, 0069-1000-02) and images of labels with the new tradename. These NDCs will not be manufactured. Only NDCs for the subsequently BLA approved tris-sucrose formulation will be produced." Labeling for CBER-Regulated Products | FDA Labeling for CBER-Regulated Products The Federal Food, Drug, and Cosmetic Act authorizes FDA to require that prescription drug labeling provides healthcare professionals and patients with adequate...
Introduction to the New Prescription Drug Labeling by the FDA A prescription drug product label (also known as a professional label, package insert, direction circular, and package circular) is a compilation of information about a product written by the... Officially on the cdc website. COMIRNITY WILL NOT BE MANUFACTURED At that time, the FDA published a BLA package insert that included the approved new COVID-19 vaccine tradename COMIRNATY and listed 2 new NDCs (0069-1000-03, 0069-1000-02) and images of labels with the new tradename. These NDCs will not be manufactured. Only NDCs for the subsequently BLA approved tris-sucrose formulation will be produced." Veterinary Labels: FDA Approved Veterinary Information from VetLabel.com Recently Updated Veterinary Product Labels and Package Inserts Simparica TRIO (Sarolaner, Moxidectin, and Pyrantel) SIMPARICA TRIO is indicated for the prevention of heartworm disease caused by Dirofilaria immitis and for the treatment and control of roundworm (immature adult and adult Toxocara canis… Zoetis Inc. 14 June 2022 51407-348-01 | Warfarin | National Drug Codes | TXT (Plain Text) The labeler code and product code segments of the National Drug Code number, separated by a hyphen. Asterisks are no longer used or included within the product code segment to indicate certain configurations of the NDC. Product Type Name: HUMAN PRESCRIPTION DRUG: Indicates the type of product, such as Human Prescription Drug or Human OTC Drug.
› article › fda-denies-requestD-tagatose Added Sugar Classification Exemption Denied by FDA May 24, 2022 · In a letter dated May 18, 2022, FDA denied a citizen petition from Bonumose LLC which requested that the Agency (1) exempt D-tagatose from classification as an “Added Sugar” and (2) allow for ... › documents › 2016/05/27Federal Register :: Food Labeling: Revision of the Nutrition ... May 27, 2016 · After rating the product's nutritional attributes, participants who viewed labels that included one of the five modified footnotes or the current footnote were asked to rate the footnote statement's understandability, usefulness, believability, and helpfulness for the following dietary tasks: Comparing products, planning a healthy diet ... Good Health Matters | Consumer Healthcare Products Association - CHPA Acetaminophen Children's Cough and Cold Medicines DXM (dextromethorphan) Monograph Reform OTC Distribution Pediatrics Medicine PSE (Pseudoephedrine) RX-to-OTC Switch Sunscreen Triclosan Dietary Supplements CBD and Hemp Oil DSHEA Immunity New Dietary Ingredients Probiotics Vitamins and Minerals Consumer Medical Devices 510 (K) Modifications Flexitol Foot and Knee Pain Relief: Details from the FDA, via OTCLabels.com FDA has not evaluated whether this product complies. Drug Facts. Active ingredient. Methyl Salicylate10% Purpose. Topical analgesic. Uses. For the temporary relief of minor aches and pains of muscles and joints associated with: ... MANUFACTURE (43251-2400), LABEL (43251-2400), ANALYSIS (43251-2400), PACK (43251-2400) Revised: 06/2022 LaCorium ...
Over the Counter Medications: FDA Approved Information from OTCLabels.com Welcome to OTCLabels.com Find the latest over the counter medication package inserts and labels directly from the approved FDA repository of SPL records, keep up with an RSS feed of all FDA over the counter medication updates, or follow a company-specific feed of over the counter medication updates from an individual manufacturer.
0078-0380-05 | Dexmethylphenidate Hydrochloride | National Drug Codes ... 0078-0380-05. The labeler code, product code, and package code segments of the National Drug Code number, separated by hyphens. Asterisks are no longer used or included within the product and package code segments to indicate certain configurations of the NDC. Package Description. 100 TABLET IN 1 BOTTLE (0078-0380-05)
Tech | Best-In-Class Information-Based Solutions and ... Navigator™ for Drug Labels helps pharmaceutical regulatory researchers ensure accurate and up-to-date drug label content. Structured Product Labeling Services for Rx, OTC and Biologics Manage product listing, establishment facility, and labeler company data across multiple teams with a secure cloud-based solution.
Senior Regulatory Specialist at Arbonne | JobEka.lk Prepare drug listings for otc drug products for fda structured product labeling (electronic submissions required by fda). Prepare product notification or pre-market approval submissions in other markets for line extensions and new product releasesThese requirements may include current markets such as canada, australia, new zealand, european ...




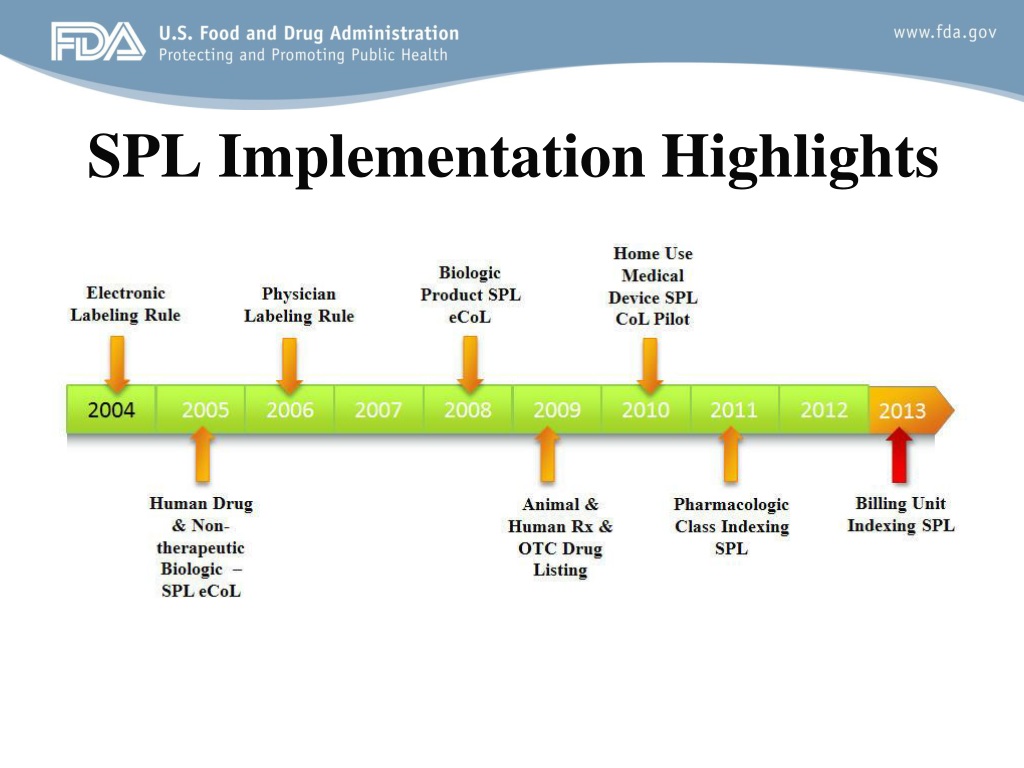



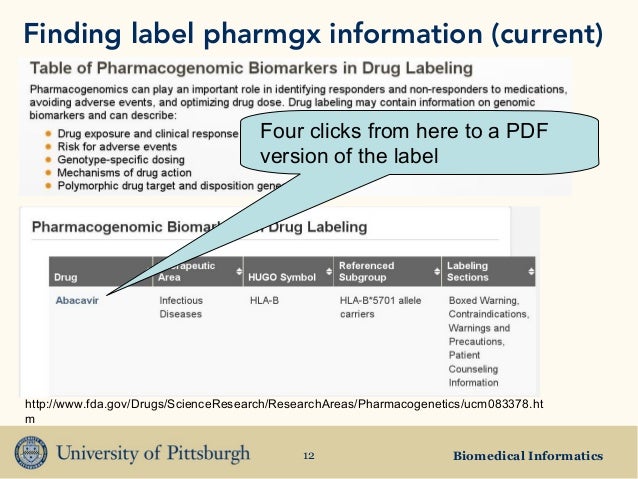

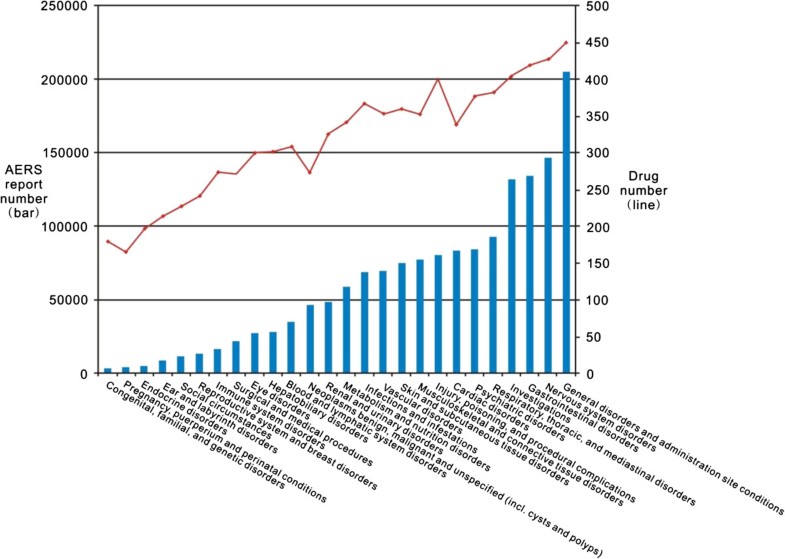


Post a Comment for "45 fda structured product labels"