44 fda health claims on food labels
Drugs vs. foods - Health claims on food labels - Food labels - Canadian ... Health claims on food labels Drugs vs. foods . Just like the term "food" (definition), the term "drug" (definition) is also defined in the Food and Drugs Act.One difference between foods and drugs is in how they are represented. If a product meets the definition of a food and is regulated as a food, the product is not permitted to carry a drug claim, unless exempted. › pet-food-labels-generalPet Food Labels - General | FDA The term "natural" is often used on pet food labels. AAFCO has developed a feed term definition for what types of ingredients can be considered “natural” and “Guidelines for Natural Claims ...
Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are...

Fda health claims on food labels
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and... A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural." Qualified Health Claims | FDA - U.S. Food and Drug Administration The process does not involve rulemaking. For more information, visit Questions and Answers: Qualified Health Claims in Food Labeling or explore the linked pages below. Qualified Health...
Fda health claims on food labels. FDA Proposes New 'Healthy' Claim on Food Labels Regulatory Compliance FDA Proposes New 'Healthy' Claim on Food Labels Sept. 28, 2022 Its food group-based approach continues prohibitions but allows salmon and nuts to be considered healthy. The FDA today (Sept. 28) issued a proposed rule to update the definition of the "healthy" claim on food & beverage packaging. 5 Understanding Food Labels and Health Claims - Maricopa Health Claims & Foods To keep companies from making false claims, the FDA provides food manufacturers' regulations in putting labels on packages that promote health. There are three levels of health claims: A health claim is supported by scientific evidence. An example is "reduces heart disease." Introduction to Food Product Claims — FDA Reader There are two types of health claims that appear on food labels and marketing. They are: Authorized Health Claims Qualified Health Claims Requirements for a Health Claim Health claims cannot be made about the diagnosis, cure, mitigation or treatment of diseases (this is a drug claim) They must be complete, truthful and not misleading. Use of the Term Healthy on Food Labeling | FDA The FDA has begun a public process to update the "healthy" claim for food labeling to be consistent with current nutrition science and federal dietary guidance. Updating the "healthy" claim...
FDA proposes voluntary 'healthy' food label claim However, foods must meet specific nutrient-related criteria to use the nutrient content claim "healthy.". On September 28, 2022, the FDA issued a proposed rule to update the definition of the nutrient content claim "healthy," which was set in 1994. The existing definition has limits for total fat, saturated fat, cholesterol and sodium ... › food › food-labeling-nutritionLabel Claims for Conventional Foods and Dietary Supplements Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ... Fda Health Claims On Food Labels FDA Proposes to Update Definition for "Healthy" Claim on … Health (7 days ago) September 28, 2022. The U.S. Food and Drug Administration today issued a proposed rule to update the definition of the nutrient content claim "healthy.". FDA Proposes to Update Definition for "Healthy" Claim on Food Labels The "healthy" claim can act as a quick signal on food package labels to help empower consumers, including those with lower nutrition knowledge, with information to identify foods that will...
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient... Structure/Function Claims - FDA Mar 7, 2022 ... If a dietary supplement label includes such a claim, it must state in a "disclaimer" that FDA has not evaluated the claim. The disclaimer must ... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (1) health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written... Questions and Answers on Health Claims in Food Labeling | FDA Health claims in food labeling are claims that have been reviewed by FDA and are allowed on food products to show that a food or food component may reduce the risk of a disease or a...
FDA proposes updates to 'healthy' claim on food packages | CNN In order to be labeled with the "healthy" claim, products would need to: Contain a certain, meaningful amount of food from at least one of the food groups or subgroups - such as fruits,...
Food Label Claims: What You Can and Can't Trust - WebMD Food Claims to Watch Out For. Some health claims on foods lack official definitions. ... FDA: "Label Claims for Conventional Foods and Dietary Supplements," "Organic on Food Labels," "Producing a ...
What You Need to Know About Health Claims on Food Labels and Dietary ... In general, health claims are statements made on food product labels or dietary supplements that boast some type of health benefit. This may seem simple, but the FDA doesn't treat every claim the same way. Label claims come in multiple forms: Health claims (which comprise of authorized health claims and qualified health claims)
› animal-veterinary › animal-food-feedsPet Food | FDA - U.S. Food and Drug Administration For more information about labeling requirements, see Pet Food Labels - General. FDA also reviews specific claims on pet food, such as “maintains urinary tract health,” “low magnesium ...
Health claims on food labels - PubMed Food and drug law requires that the ingredients in most foods be disclosed on their labels, but until recently there was no requirement that nutrition information be provided. ... Labeling and Education Act of 1990 (NLEA), passed on November 8, 1990, mandated the Food and Drug Administrati … Health claims on food labels Mil Med. 1994 Mar;159 ...
Health Claims on Food Labels | LegalMatch In short, yes. A health claim must be approved by the Food and Drug Administration ("FDA") before the manufacturer is allowed to put the health claim on one of their food products. In general, there are two ways in which a manufacturer can obtain FDA approval: Scientific Data and Evidence: The most common way in which a manufacturer can ...
FDA perspectives on health claims for food labels - PubMed FDA perspectives on health claims for food labels Authors J Craig Rowlands 1 , James E Hoadley Affiliation 1 Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, College Park, MD 20740, USA. JCRowlands@Dow.com PMID: 16480811 DOI: 10.1016/j.tox.2005.10.023 Food Labeling* Legislation, Food* Nutritive Value Research Design
› food › food-labeling-nutritionFood Allergies | FDA - U.S. Food and Drug Administration Oct 20, 2022 · People with food allergies should read labels and avoid the foods they are allergic to. The law requires that food labels identify the food source of all major food allergens used to make the food.
Fda Health Claims For Food Questions and Answers on Health Claims in Food … Health (8 days ago) 4. Has the FDA ever revoked an authorized health claim? The FDA has authorized 12 health claims since 1990. On October 31, 2017, the agency issued a proposed rule to revoke the regulation that
› consumers › consumer-updatesIs It Really 'FDA Approved'? | FDA - U.S. Food and Drug ... May 10, 2022 · The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and ...
Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ...
Everything you need to know about Health Claims on Food Labels The authorized health claims by the FDA must have significant scientific agreement among qualified experts to support the scientific evidence for a substance - disease relationship. The FDA has approved 12 health claims on food labels such as sodium and hypertension; fiber-containing grains, fruits and vegetables and cancer; and more.
Auburn food historian explains new FDA guidelines for 'healthy' food labels Last week, the U.S. Food and Drug Administration, or FDA, updated its criteria for foods labeled "healthy." The proposed change is based on current nutrition science and prioritizes healthy dietary patterns, continuing from the FDA's overhaul of the Nutrition Facts panel in 2016.
FDA Proposes Updated Definition of 'Healthy' Claim on Food ... Sep 28, 2022 ... Today, the U.S. Food and Drug Administration proposed updated criteria for when foods can be labeled with the nutrient content claim ...
Health claims on food labels - Food labels - Canadian Food Inspection ... A health claim is any representation in labelling or advertising that states, suggests, or implies that a relationship exists between the consumption of a food and health. All aspects of food labels and advertisements contribute to the overall impression made by a food product, including health claims. For this reason, health claims are also ...
Definitions - Health claims on food labels - Food labels - Canadian ... Claims that refer to the specific beneficial effects that the consumption of a food or food constituent has on normal functions or biological activities of the body. Such claims relate to a positive contribution to health or performance. For example, " [Naming the food or food constituent] promotes regularity or laxation".
› food › guidance-regulation-food-andFood/Dietary Supplement Guidance and Regulatory Information Oct 20, 2022 · WITHDRAWN Acidified and Low-Acid Canned Foods: (DRAFT) Submitting Form FDA 2541 (Food Canning Establishment Registration) and Forms FDA 2541d, FDA 2541e, FDA 2541f, and FDA 2541g (Food Process ...
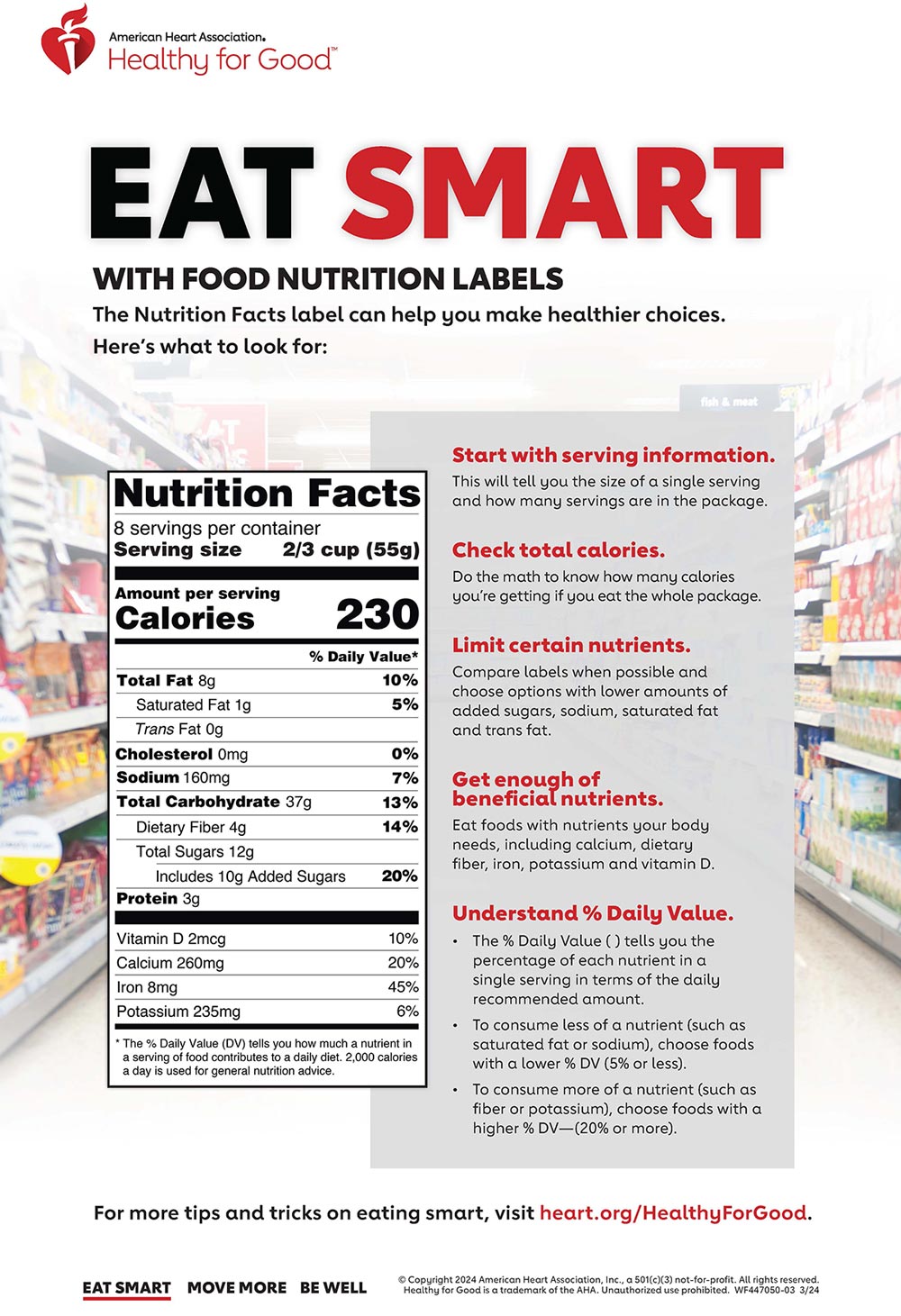
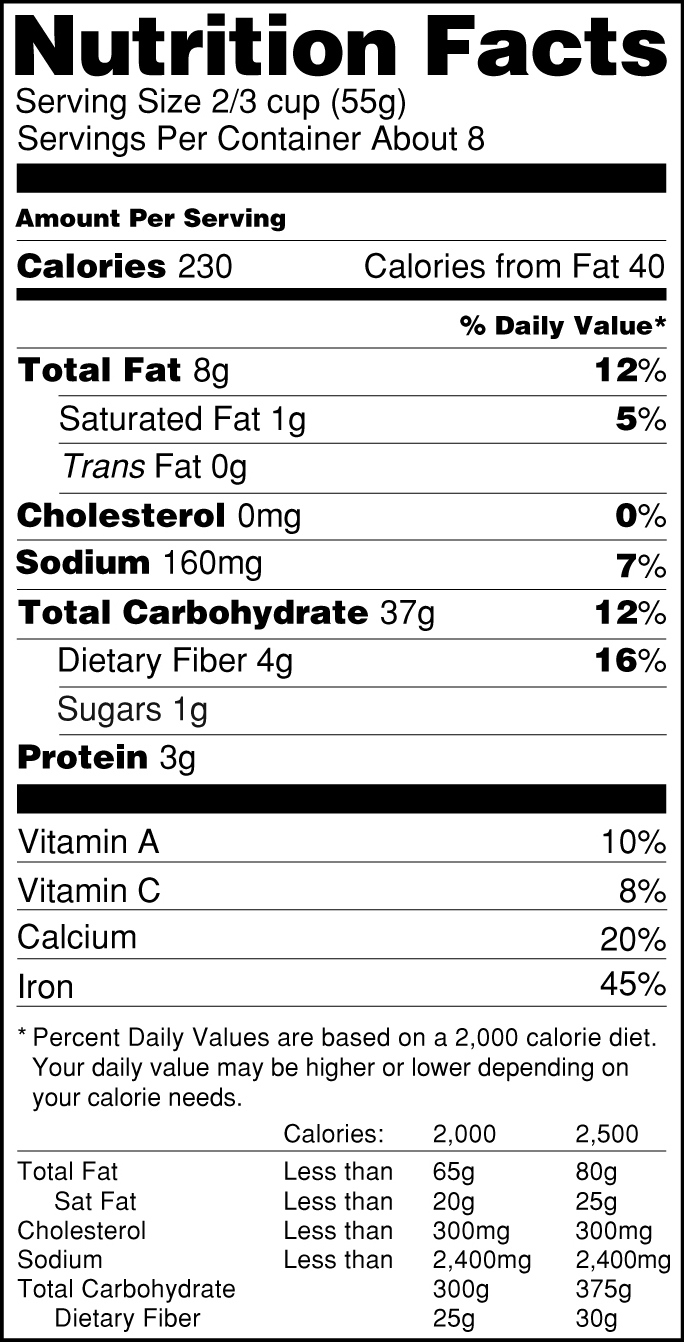
› food › new-nutrition-facts-labelDaily Value on the New Nutrition and Supplement Facts Labels Feb 25, 2022 · However, they are required to list any vitamins and minerals that are added to the food or if a statement is made on the package labeling about their health effects or the amount contained in the ...
Qualified Health Claims | FDA - U.S. Food and Drug Administration The process does not involve rulemaking. For more information, visit Questions and Answers: Qualified Health Claims in Food Labeling or explore the linked pages below. Qualified Health...
A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural."
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and...
































:max_bytes(150000):strip_icc()/juicy-juice-no-sugar-400x400-b1fb04c46e9e4c8392ce8881614c021a.jpg)


Post a Comment for "44 fda health claims on food labels"